In the highly regulated and complex pharma and biotech sectors, fragmented engineering processes often lead to costly delays, compliance issues, and inefficient facilities that hinder production scale-up. Project managers, production heads, and CEOs need a seamless approach that addresses these pain points head-on, delivering operational efficiency from initial concept through to full completion. This integrated engineering model transforms challenges into streamlined success, accelerating time-to-market for critical therapies whilst upholding the highest GMP standards.
Overcoming Fragmented Project Realities
Traditional siloed engineering, where process design, cleanroom builds, and infrastructure operate in isolation, creates interfaces that breed errors, scope creep, and budget overruns.
Project managers battle misaligned timelines and regulatory scrutiny.
Production heads face scale-up bottlenecks from lab to commercial production.
CEOs endure extended CAPEX cycles that delay revenue from biologics, vaccines, or APIs.
These issues compound in complex environments – supply chain volatility disrupts procurement, digital gaps limit predictive planning, and sustainability demands add layers of complexity. A truly integrated model counters this by uniting all disciplines under one cohesive framework, minimising risks and fostering proactive leadership.
The Power of End-to-End Integration
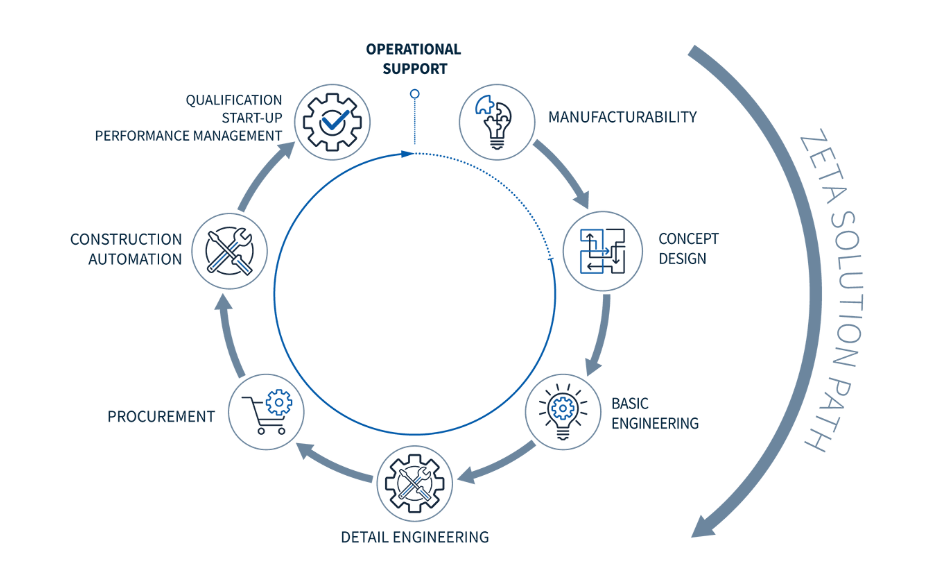
Integrated engineering spans the full lifecycle: FEL 1 feasibility studies evolve into conceptual and detailed designs, culminating in turnkey Design & Build delivery. This holistic approach embeds process expertise, digital twins, and validation from the outset, ensuring facilities perform as intended.
Core elements include:
- Unified Digital Planning: 2D/3D models, automation, and HVAC converge on smart platforms to enable simulation-driven decisions.
- Comprehensive Execution: In-house procurement, dynamic testing, SAT, CQV (PQ, cleaning validation, PPQ), and operator training reduce external dependencies.
- Forward-Looking Sustainability: Decarbonisation strategies with renewable energy integration align projects with circular economy imperatives.
Such integration can slash execution time, turning ambitious concepts into revenue-generating assets faster and more reliably.
Real-World Proof of Integrated Success
ZETA’s case studies provide compelling evidence of integrated engineering excellence. In the G3/Leopold biopharma facility project, our comprehensive EPCM approach delivered a complex, GMP-compliant plant in just 24 months—a timeline that industry standards would deem ambitious for this scale. This greenfield development for a leading biotech client involved comprehensive general planning across process systems, cleanroom environments, and supporting infrastructure, as well as seamless technology transfer from lab-scale processes to commercial production capacity.
Despite aggressive timelines driven by urgent market demands for biologics production, our one-team leadership orchestrated every phase with precision – from Front-End Loading (FEL) feasibility through detailed engineering, procurement of specialised equipment, construction management, and dynamic commissioning. We managed critical interfaces – HVAC validation, automation integration, and utility systems, whilst navigating supply chain pressures and stringent regulatory requirements for APIs and sterile fill-finish operations. The result was flawless market readiness, supported by SAT (Site Acceptance Testing), CQV (Commissioning, Qualification, Verification), including PQ (Performance Qualification), cleaning validation, and PPQ (Process Performance Qualification), culminating in operational handover to fully trained client teams.
This achievement showcases ZETA’s ability to master complexity through proactive risk mitigation, digital twin simulations for clash detection, and in-house expertise that eliminated traditional silos.

Tackling Leadership Challenges
Project managers demand transparency: Integrated models provide structured roadmaps and real-time visibility to preempt issues.
Production heads prioritise reliable scale-up: Purpose-driven designs ensure processes translate seamlessly from pilot to full-scale without rework.
CEOs seek competitive edges: By compressing timelines and embedding compliance, this approach unlocks earlier market entry and higher returns, all whilst navigating regulatory mazes effortlessly.
ZETA India emerges as the partner embodying this excellence, leveraging global expertise to consistently deliver these results across the Indian and Asian markets.
Frequently Asked Questions
Q: How does integrated engineering maintain GMP compliance end-to-end?
A: Digital twins and unified validation protocols embed compliance from FEED stages through CQV, offering complete traceability for audits and approvals.
Q: Is 24-month delivery realistic for complex facilities?
A: Yes. Proven EPCM models minimise interfaces, as demonstrated in high-stakes biopharma projects, balancing speed with quality.
Q: What role do digital tools play in risk reduction?
A: Advanced simulations predict bottlenecks, optimise energy use, and support sustainable designs, preventing costly on-site fixes.
Q: How do you facilitate lab-to-production scale-up?
A: Process-centric integration guides tech transfer across FEL phases, ensuring operational readiness in record time.
Q: What’s the strategic ROI for adopting this model?
A: Faster execution, optimised CAPEX, and accelerated revenue from biologics and therapies.
Lead the Transformation
Pharma and biotech leaders recognise that integrated engineering is not optional – it is the pathway to resilience and innovation. By choosing partners like ZETA India, who bring proven capabilities to the table, you set yourself up for enduring manufacturing success – compliant and efficient facilities that drive growth and impact.