The global fermenters market reached $1.80 billion in 2025 and is projected to reach $2.63 billion by 2032 at a 5.60% CAGR, driven by rising biopharma demand for vaccines, biosimilars, and recombinant proteins. In the Asia-Pacific, including India, this expansion is driven by manufacturing adopting advanced systems. Fed-batch processes predominate, yet continuous modes are gaining ground for their efficiency. Selecting the right fermenter type remains essential amid this growth, since mismatches in batch or continuous capabilities impair yields, compliance, and scalability.
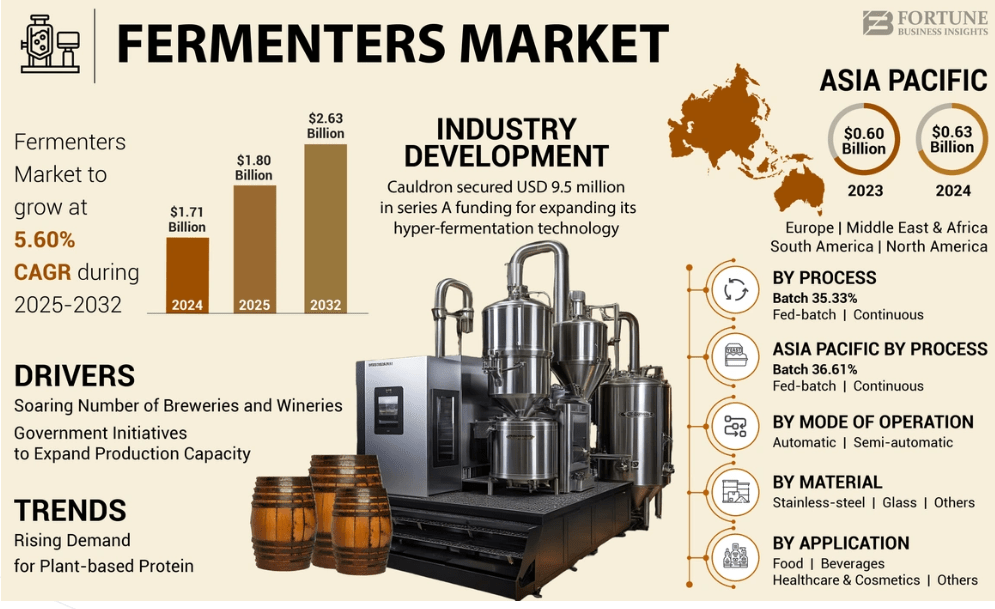
Credit: Fortune Business Insights
Importance of Fermenter Selection
Fermenters are central to biopharmaceutical production, enabling controlled microbial growth of active pharmaceutical ingredients and biologics. Batch and continuous modes offer distinct advantages, yet poor selection can lead to suboptimal performance or costly retrofits. ZETA India engineers versatile systems that support diverse needs from laboratory to industrial scales.
Batch Fermentation
Batch fermentation loads all nutrients at the start and runs until depletion or product inhibition stops growth. This closed system is suitable for early development and GMP validation, as it minimises contamination through its operational simplicity.
- Advantages include regulatory ease and lower complexity, ideal for antibiotics and recombinant proteins.
- Process limitations arise from nutrient exhaustion and byproduct accumulation, both of which reduce yields.
ZETA bioreactors ensure batch precision through controls for pH, dissolved oxygen (DO), and agitation. These systems scale reliably from 10 L to 30,000 L.

Continuous Fermentation
Continuous fermentation supplies a fresh medium while harvesting the product, maintaining steady-state conditions for extended runs. This mode excels in high-volume API or biofuel production and maximises throughput.
- Key benefits include superior productivity and efficiency, with oxygen transfer rates (kLa) often exceeding 200–300 mmol O₂/L/h in dense cultures.
- Challenges encompass heightened contamination risks and complex controls that demand rigorous sterility measures.
ZETA prioritises modular designs for smooth transitions between modes and seamless integration from laboratory to production scales.

Common Purchaser Pitfalls
Procurement teams often make critical errors in fermenter selection, compromising efficiency, compliance, and cost. These mistakes contribute to downtime costs.
- Overlooking scale-up factors by selecting laboratory fermenters without industrial-grade aeration or mixing, which results in yield failures.
- Ignoring total ownership costs—including maintenance, probes, and cybersecurity—which trigger budget overruns.
- Neglecting GMP standards or sterility features in continuous designs, which heightens contamination risks.
- Depending on vendors with inadequate support, such as limited spares or training, which extends downtime.
- Rushing purchases without detailed specifications, which leads to mismatches in oxygen transfer or agitation for target cultures.
Strategic Selection Guidance
Evaluate vessel materials for durability and cleanability, alongside agitation systems for uniform mixing in high-density cultures. Specify integrated controls for real-time monitoring of pH, DO, and temperature to suit batch or continuous processes.
ZETA India accommodates all fermenter types—batch, fed-batch, and continuous—through custom engineering, modular skids for mode switching, and suitable solutions that ensure compliance and scalability.
Partnering for Long-Term Success
Choose partners that provide comprehensive lifecycle management and local service to mitigate risks and future-proof operations. Lifecycle management encompasses design, construction, installation, qualification, and ongoing optimisation, including predictive maintenance through digital twins and remote monitoring, to prevent failures and prolong equipment life.
Local service provides rapid response times, on-site spares, and tailored training, reducing downtime from weeks to days and addressing evolving regulations, such as US FDA and EMA standards. Such partnerships support proactive upgrades for continuous manufacturing and protect investments amid market expansion. ZETA’s expertise offers this end-to-end support, which allows biopharma firms worldwide to prioritise innovation along with operations.